Science Memes
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz
Memes
Miscellaneous
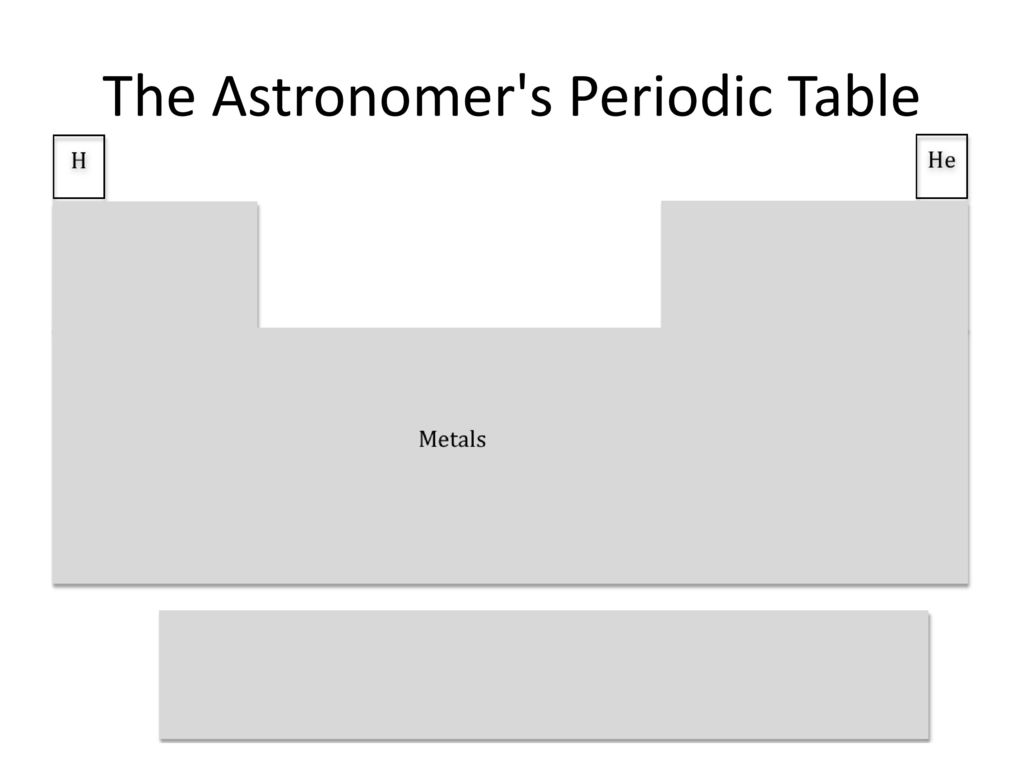
Do you know what happens to hydrogen when the temp drops below 14K?
Yeah. Metal.
Metallic hydrogen may also make up parts of Jupiter's core.
Metallic or solid? Those are two different things, and depending on the answer, i will be going down a knowledge rabbit hole
Metals are crystal lattices with delocalized electrons.
That's fucking badass
That's hard af
Doesn't it also need to be under immense pressure? I don't think low temperature alone is enough.
Yeah, I think that may be the case.
🤘
I'm confused, that's just a normal periodic table.
Found the astronomer.
what? no, a normal periodic table has oxygen and carbon too!
Found the organic chemist
i mean, i think most chemists are organic
few are free range though
Physicists are notorious for approximating, and astronomers are even worse. But there are some subfields where they care about being more precise, and you maybe break the periodic table into a handful of elements plus alphas. And there's that one or two people getting exquisite spectral resolution and signal-to-noise on a few stars and measuring the abundance of Technetium or whatever.
It's why I fucking love astrophysics. There's so much handwaving because so much information is observed.
But without the handwaving you can't find crazy ass things like nuclear fusion being behind the power of stars. You find these really big numbers everywhere that make the "normal stuff" negligible.
It not that the precision isn't important, it's just not always relevant at particular scales, like the scale of space.
Plutonium is not a real element.
It's a dwarf element.
Plutonium can be on the periodic table but we do not grant it the rank of element.
What about metallic hydrogen in the core of planets?
"Wait, they're ALL metals?"
"Always have been."
Funnily enough, probably not a metal according to astronomers.
Iodine is a transition metal I will die on this hill.
Care to defend your position? Iodine is certainly not in the d-block...
The intended joke is that hypervalent iodine compounds like Dess-Martin periodinane flip between different oxidation states like you often see for transition metals. As an example, the mechanism usually drawn for oxidations by DMP is similar to those drawn for PCC/Jones reagent, where the electrons removed from the substrate are "banked" at the metal center. Obviously, redox chemistry is not at all limited to transition metals, but I am often surprised at iodine's propensity to engage in it. A lot of research over the past decade or two has also developed redox catalysis with these reagents, reactivity which is commonly (though again not always) the purview of transition metals.
\m/
And if you ask a cosmologist what the universe is made of, they go "Well, there's a lot of dark matter, and even more dark energy. And then there's a tiny bit of some matter or something idk lol."
Read that as cosmetologist and was thoroughly confused.